

In 2008, an article in Molecular Therapy ( Mol Ther 16: 590−598) described a new generation of self-inactivating (SIN) vector that lacks all enhancer–promoter elements of the gammaretroviral LTR U3 region. Interestingly, gene therapy for ADA-SCID, using a similar gammaretroviral vector, was not plagued with leukemia, suggesting contributions by the vector and the specific disease/transgene to the malignancy. Murine gammaretroviral vectors have a tendency to integrate in the promoter region of transcriptionally active genes and, in the case of patients with SCID-X1, favored clonal dominance for cells with integration near, in particular, the T-cell oncogene Lmo2. An argument has arisen in the gene therapy field about the relative contributions of the transgene (which in SCID-X1 provides a substantial growth advantage over untransduced cells) and the vector. Gene therapy, using the patient's own cells, avoids the need for matched donors as well as GvHD and other bone marrow transplant–related complications. Transplants from unrelated or haploidentical donors are complicated by graft-versus-host disease (GvHD), side effects of chemotherapy, and incomplete lymphoid reconstitution. However, a matched family donor is available only for approximately 30% of patients.
When evaluating the success of gene therapy for SCID-X1, one must keep in mind that the disorder has very poor prognosis without treatment and results in death from infectious disease in the first year of life. In this regard, gene therapy appears to offer a genuine therapeutic alternative to mismatched allogeneic hematopoietic stem cell transplantation (HSCT). The results from the London study show similar effects on restoration of immunity, and only one child has developed leukemia. Remarkably, four of the seven have not required immunoglobulin infusions although long-term transduction of B cells was not observed. The seven children with functional immune systems have responded to vaccination and live fairly normal lives. Gene-corrected T cells have persisted for more than 10 years. The other three were successfully treated with chemotherapy and were among seven children who exhibited long-term immune reconstitution.
Gene therapy for scid trial#
One of the four children who developed leukemia in this trial had died. The findings of long-term follow-up on nine boys from the French trial, now 8–11 years old, were recently published.
These events led to the development of highly sensitive polymerase chain reaction techniques to detect viral integration sites and improve vector design, thereby substantially increasing safety of clinical trials based on integrating vectors.Īt the same time, during the period of continual attention to the leukemia cases, including by news media, the tremendous success achieved by these trials seemed almost unnoticed. However, the approach experienced a major setback when 5 of the 20 boys developed leukemia due to insertional mutagenesis and activation of an endogenous proto-oncogene.
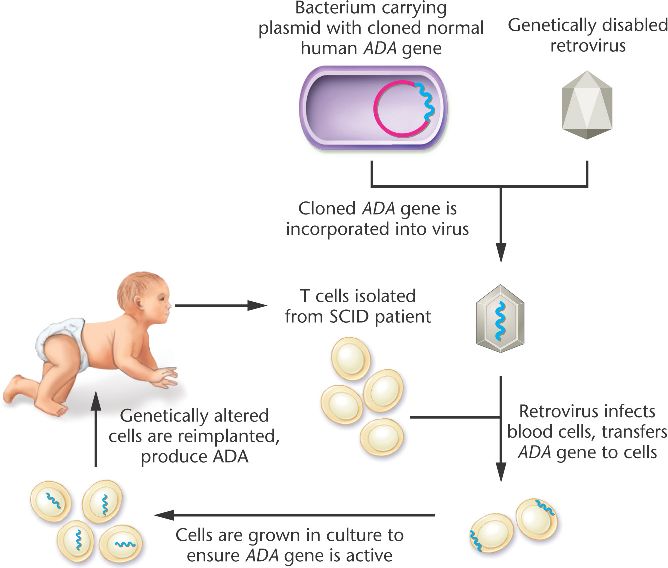
Gene therapies were performed in two clinical trials in France and the United Kingdom in a total of 20 boys. Retroviral gene transfer of the common cytokine receptor γ-chain, required for the function of multiple cytokine receptors, to bone marrow–derived hematopoietic stem and progenitor cells reconstituted development of functional T cells and thereby also partially restored the ability of the affected boys to mount proper B-cell responses. When reported in 2000, gene therapy for X-linked severe combined immune deficiency (SCID-X1) represented a first clear success in clinical gene therapy, providing a tremendous boost for the field.


 0 kommentar(er)
0 kommentar(er)
